Supply Chain Integration with PragLife: Orchestrating Seamless Connectivity Across the Cell & Gene Therapy Ecosystem
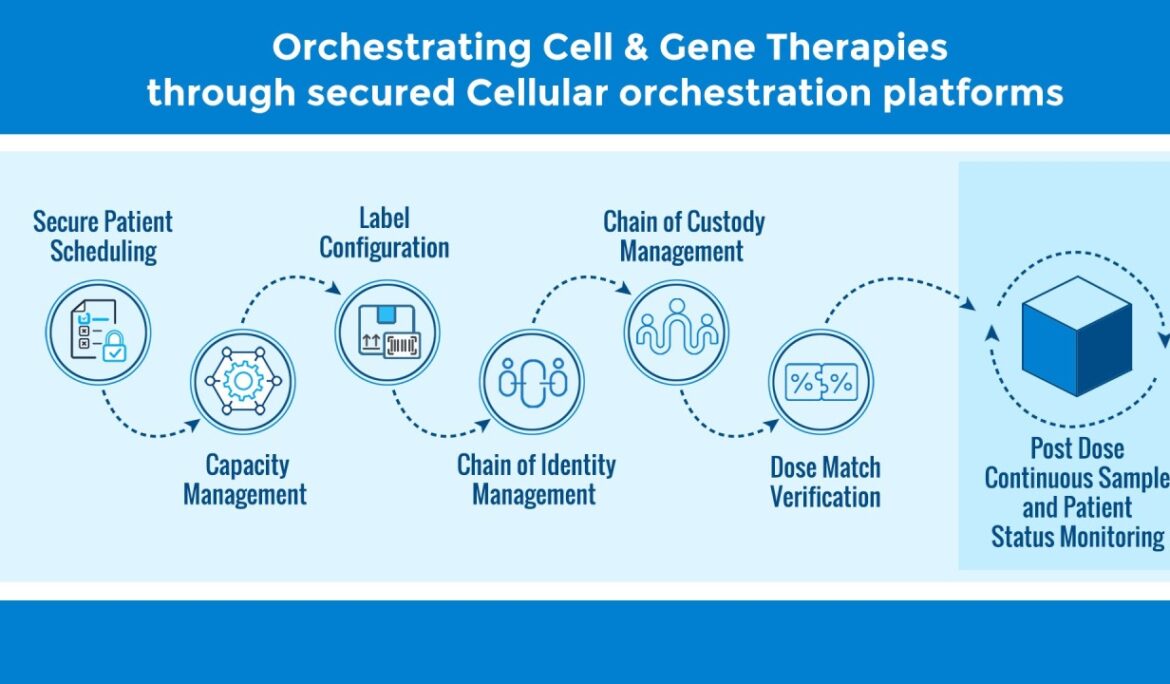
In cell and gene therapy manufacturing, the difference between a life-saving treatment reaching a patient on time and a critical delay often comes down to one factor: supply chain integration. The complexity is staggering. A single CAR-T therapy journey involves coordinating patient enrollment, apheresis scheduling, cryopreservation, transportation across multiple time zones, GMP manufacturing, quality control, final product delivery, and patient infusion, all while maintaining perfect Chain of Identity (COI) and Chain of Custody (COC) at every touchpoint. Manual coordination across these stakeholders is not just inefficient. It’s a patient safety risk. The Integration Imperative in Personalized Therapies Cell and gene …
How Cellular Orchestration Platforms Handle the Diversity of Cell & Gene Therapies
The cell and gene therapy landscape in 2025 presents an unprecedented challenge: the pipeline contains over 4,000 candidates, half of them gene therapies – according to the American Society of Gene & Cell Therapy (ASGCT) and Citeline’s Q3 2024 report. Each therapy brings unique requirements for manufacturing, tracking, and delivery. From CAR-T cells requiring precise temperature control to allogeneic NK cells demanding different storage conditions, the diversity is staggering. Yet within this apparent chaos lies an opportunity for organizations that can master the complexity through intelligent software orchestration. The Scale of Diversity Challenges Modern Organizations Cell and gene therapies present …