Why Modular Software Architecture Is Becoming Essential for Cell Therapy Operations
The cell and gene therapy industry operates under a unique set of pressures. Every organization has distinct workflows, different regulatory requirements across regions, and specific operational processes that have been validated over years. Yet many software solutions on the market today force companies into a difficult choice: adopt a rigid, one-size-fits-all platform, or piece together multiple disconnected systems that create data silos and compliance gaps. Neither option serves the patient-centric mission that drives this industry forward. The Challenge: Complexity Without Flexibility GMP manufacturing environments are inherently complex. A CDMO managing multiple client programs needs different capabilities than a biotech running …
Cell and Gene Therapy Trends for 2026: Navigating the Evolution of Advanced Therapeutics
By the end of 2026, the way we manufacture cell and gene therapies will look fundamentally different than it does today. Not because of scientific breakthroughs alone, but because operational realities are forcing a transformation that’s long overdue. The cell and gene therapy (CGT) landscape stands at a pivotal moment. With over 3,700 therapies in clinical and preclinical development and 76 products already launched globally, 2026 promises to be a defining year where innovation meets operational reality. For patient-centric organizations, CMOs, and CDMOs, understanding these emerging trends is not just about staying current, it’s about positioning your operations for sustainable …
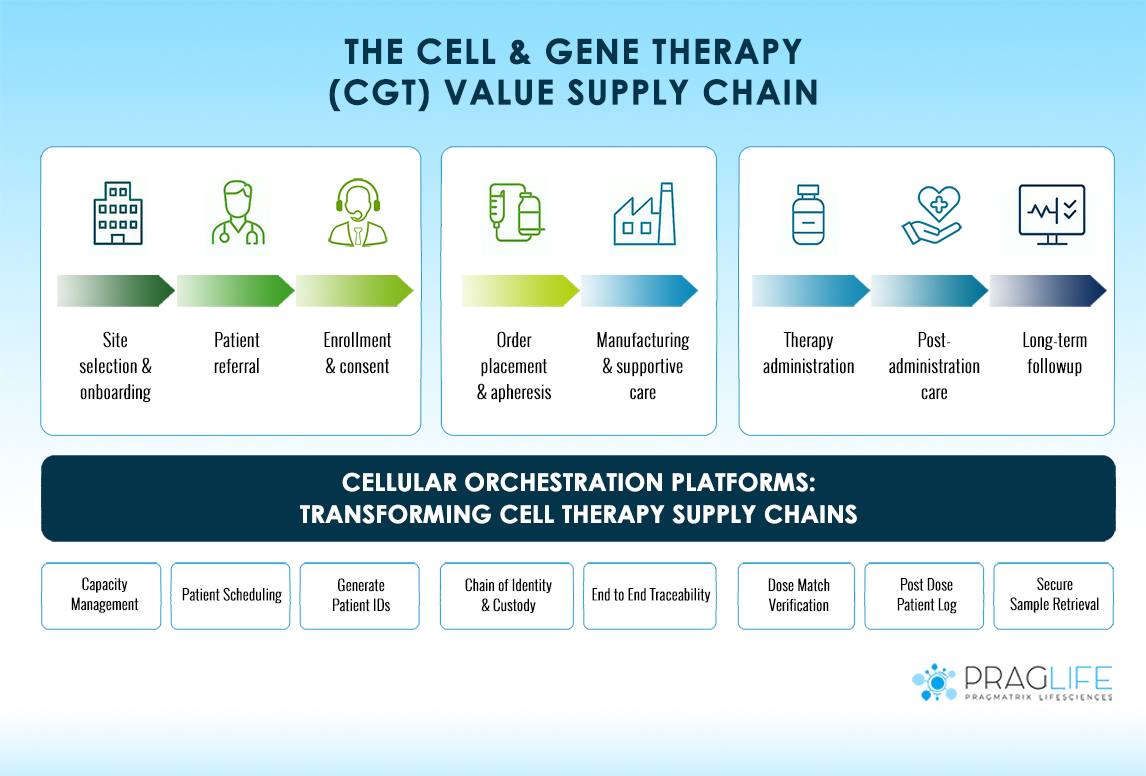
Why Cell Therapy Supply Chains Need Cellular Orchestration Platforms
The cell and gene therapy (CGT) landscape has evolved dramatically over the past decade. With over 3,000 clinical trials worldwide and commercial therapies reaching patients at unprecedented speed, the industry faces a critical challenge orchestrating increasingly complex supply chains that span continents and involve multiple stakeholders. Unlike traditional pharmaceuticals manufactured in standardized batches, cell therapies follow a personalized model where each treatment is unique to one patient. This fundamental difference creates cellular orchestration challenges that traditional supply chain management approaches simply cannot address. The Complexity Challenge in Cell Therapy Supply Chains Cell therapy supply chains operate fundamentally differently from conventional …
7 Critical Ways Sample Inventory Management Systems Enhance Biological Sample Traceability and FDA Audit Readiness
In the highly regulated world of cell and gene therapy, maintaining impeccable sample traceability and audit readiness isn’t just good practice – it’s essential for patient safety, regulatory compliance, and therapeutic success. As biological samples become increasingly valuable and personalized therapies more common, the stakes for error-free inventory management have never been higher. The Growing Challenges of Biological Sample Management According to recent industry data, specimen loss rates hover around 0.002%, which may seem small until you realize this translates to thousands of mishandled samples monthly across healthcare systems. Each lost sample represents not just a logistical failure, but potentially …
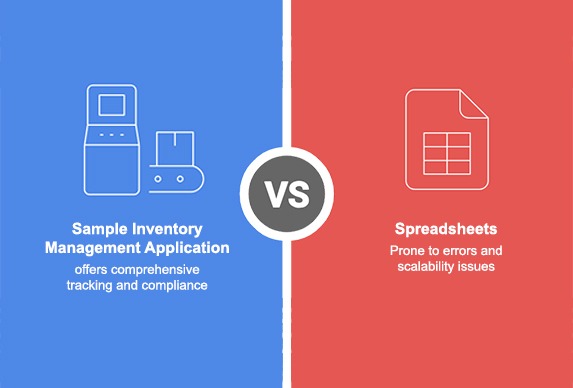
Spreadsheets Vs. Sample Inventory Management Systems: A Comparative Guide for Managing Biological and Cell Therapy Sample Data
In the highly regulated world of life sciences and cell therapy, proper inventory management of biological samples isn’t just good practice it’s critical for patient safety, regulatory compliance, and operational success. Organizations working with biological materials face unique challenges that demand robust solutions beyond what conventional tracking methods can offer. This guide provides a thorough comparison between traditional spreadsheet-based inventory management and specialized sample storage inventory systems designed specifically for GMP-regulated environments. Understanding these differences is crucial for organizations seeking to enhance their sample management processes while maintaining FDA compliance. The Current Landscape of Sample Management in Life Sciences Many …
How a Sample Inventory Management System Supports Scaling from Phase I to Commercialization
In the world of cell and gene therapy, growth is both the goal and the challenge. Early-stage biotech companies begin with a handful of samples, managed in spreadsheets and tracked manually. But what happens when your promising therapy enters Phase II, scales to multi-site trials, and eventually moves toward commercialization? The complexity of managing biological and cell therapy samples grows exponentially, and manual processes simply can’t keep up. This is where a scalable sample inventory system, implemented step by step becomes essential for managing biological and cell therapy samples. In this use case deep dive, we explore how the right …
10 Must-Follow Regulatory Compliance Checks for Storing Biological and Cell Therapy Samples in 2025
In the rapidly evolving landscape of cell and gene therapies, maintaining regulatory compliance for biological sample storage isn’t just good practice, it’s essential for patient safety, product integrity, and business continuity. As regulatory frameworks continue to evolve in response to technological advancements and emerging therapies, organizations must stay vigilant about compliance requirements and how to implement a validated storage system. The stakes couldn’t be higher. A single compliance failure can result in compromised patient safety, regulatory actions, financial penalties, and irreparable damage to reputation. For cell therapy CDMOs, biobanks, and other patient-centric organizations, understanding and implementing these critical compliance checks …
CASE STUDY : Partnering with NYBC to Transform Their Cord Blood Storage and Distribution
A case study demonstrating how Pragmatrix’s GMP-compliant sample inventory management solutions helped the New York Blood Center (NYBC) optimize their cord blood storage and distribution while maintaining strict FDA regulatory compliance Building the Infrastructure for NYBC’s Life-Saving Cord Blood Program For over five decades, the New York Blood Center (NYBC) has played a critical role in healthcare across New York and New Jersey. As a non-profit organization committed to public health, NYBC delivers essential blood products, transfusion services, and public health research to support a population of over 20 million people. Central to this mission is the National Cord Blood …
Step-by-Step Guide to Implementing Sample Inventory Management System for Managing Biological and Cell Therapy Samples
Across the highly regulated landscape of life sciences, properly managing biological and cell therapy samples isn’t just good practice, it’s essential for regulatory compliance, patient safety, and research integrity. Yet many organizations continue to rely on manual inventory systems that create significant risks and inefficiencies. This comprehensive guide walks through the challenges of manual inventory systems, highlights the key benefits of sample inventory management systems, and offers a clear, actionable 7-phase implementation roadmap. The Limitations of Manual Sample Inventory Traditional inventory management for biological samples typically relies on spreadsheets, paper logs, or basic database systems. These approaches present several critical …
Implementing Barcodes in GMP Biologics: Overcoming Challenges & Choosing the Right Software
The implementation of barcodes in GMP (Good Manufacturing Practice) environments for patient-centric biologics handling firms can seem daunting. While the benefits are undeniable, the transition often presents challenges. Let’s delve into the practical difficulties and how the right software can ease this process. Challenges of Barcode Implementation in GMP Biologics Complex Workflows: Biologic manufacturing processes are often intricate, involving multiple stages and numerous materials. Mapping these workflows into a barcode system can be complex and time-consuming. Data Integrity Concerns: Ensuring accurate data capture and preventing errors is crucial in GMP environments. Barcode systems must be reliable and robust to maintain …