How Cellular Orchestration Platforms Handle the Diversity of Cell & Gene Therapies
The cell and gene therapy landscape in 2025 presents an unprecedented challenge: the pipeline contains over 4,000 candidates, half of them gene therapies – according to the American Society of Gene & Cell Therapy (ASGCT) and Citeline’s Q3 2024 report. Each therapy brings unique requirements for manufacturing, tracking, and delivery.
From CAR-T cells requiring precise temperature control to allogeneic NK cells demanding different storage conditions, the diversity is staggering. Yet within this apparent chaos lies an opportunity for organizations that can master the complexity through intelligent software orchestration.
The Scale of Diversity Challenges Modern Organizations
Cell and gene therapies present unique challenges that complicate adoption at scale, including individualized manufacturing processes, stringent handling and storage requirements, and complex reimbursement models. The challenge extends far beyond simple logistics.
Consider the spectrum of therapies currently in development:
Cell Type Variations:
- T-cells: Require specific activation protocols and precise temperature maintenance throughout manufacturing
- NK cells: Well suited for universal cell therapy products due to their homogeneity and minimal MHC matching requirements
- Dendritic cells: Demand specialized handling protocols and custom preparation methods
- Stem cells: Need different expansion techniques and quality control measures
Starting Material Complexity:
- Blood-derived products requiring immediate processing
- Bone marrow samples with extended viability windows
- Tissue samples demanding specialized preservation methods
Manufacturing Approaches:
- Autologous therapy uses a patient’s cells, minimizing immune rejection risk
- Allogeneic cells could be scaled for much larger patient populations if immune rejection could reliably be overcome
Why Traditional Software Falls Short
Most healthcare software systems were designed for standardized products with predictable workflows. Cell and gene therapies shatter these assumptions. Manual order processes are getting more complicated, with some having multiple cycles, several samples, or parts of the process tracking data instead of physical products.
The fundamental issue isn’t just complexity – it’s unpredictability. Each therapy brings its own set of requirements:
- Different temperature storage needs
- Varying processing timelines
- Unique regulatory documentation requirements
- Specialized shipping protocols
- Custom quality control measures
Organizations attempting to manage this diversity through spreadsheets or rigid software systems face inevitable bottlenecks, compliance risks, and scaling limitations.
How PragLife Masters Therapeutic Diversity
PragLife approaches this challenge through intelligent configurability rather than rigid customization. Instead of building separate systems for each therapy type, PragLife’s modular platform adapts to diverse requirements through smart configuration, ensuring FDA compliance and GMP standards across all therapy types.
PragLife’s Configurable Components Handle Diversity
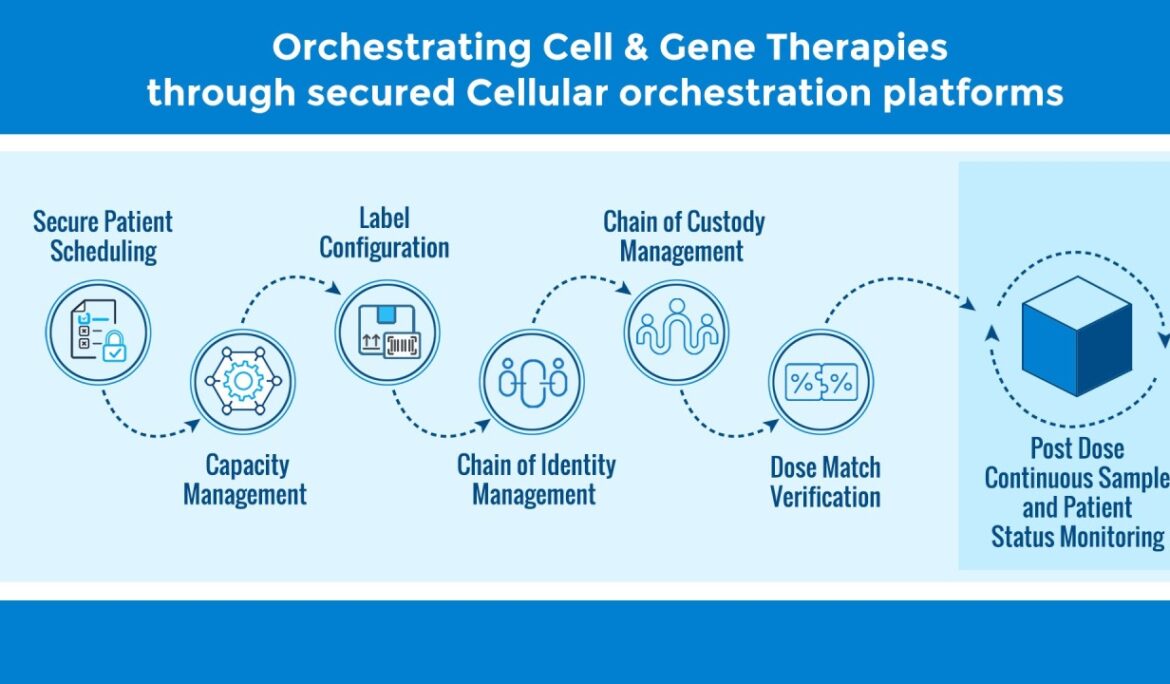
Process Orchestrator: PragLife’s Process Orchestrator empowers organizations to tailor workflows with precision, configuring processes in alignment with Standard Operating Procedures (SOPs) and unique therapy requirements for each cell type.
Universal Scheduler: Beyond basic scheduling, PragLife’s Universal Scheduler adapts to unique workflows -whether managing T-cell activation timelines, NK cell processing windows, or dendritic cell preparation schedules.
Label Configurator: Design custom labels for different therapy types with PragLife’s intuitive Label Configurator. From cryogenic storage labels for stem cells to temperature-sensitive indicators for CAR-T products, the system adapts to each therapy’s specific requirements.
Adaptable Chain of Identity (COI) Management
Chain of Identity tracking becomes critical when managing diverse therapy types. PragLife’s configurable platform adjusts COI protocols based on:
- Therapy classification (autologous vs. allogeneic)
- Starting material type (blood, bone marrow, tissue)
- Processing requirements and timelines
- FDA regulatory mandates and GMP standards
Flexible Chain of Custody (COC) Protocols
Different cell types demand different custody procedures. PragLife accommodates these variations by allowing organizations to configure custody protocols that match specific therapy requirements while maintaining compliance across all therapy types.
PragLife Apps: Tailored Solutions for Diverse Needs
PragLife offers pre-built, configurable applications that handle therapeutic diversity:
Electronic Batch Record: Seamlessly converts different therapy protocols into user-friendly digital screens with built-in validations and automated calculations specific to each cell type.
Storage Inventory System: Manages diverse bio-specimens from blood products to stem cells, all within secure cryopreservation environments, with configuration options for different storage requirements.
CliniTrack: Handles clinical trials across multiple therapy types, managing everything from CAR-T patient enrollment to NK cell collection scheduling.
Production Scheduler: Coordinates complex procedures involving different cell types, manufacturing timelines, and resource allocation across diverse therapy protocols.
Real-World Impact: Managing Complexity at Scale
With 2,093 gene therapies and 885 non-genetically modified cell therapies in various development stages according to ASGCT data, organizations need systems that scale with this growth while maintaining precision.
PragLife’s approach focuses on several key areas:
Workflow Orchestration: The platform connects patient journeys with therapy workflows, regardless of cell type or manufacturing complexity.
Electronic SOPs and Automation: Organizations maintain FDA compliance across diverse therapy types while reducing manual intervention points.
Integrated Barcode Support: PragLife’s native barcode and RFID capabilities track diverse products with different handling requirements seamlessly.
The Economic Reality of Diversity
Most advanced therapies are priced at over USD 1 million, making widespread access a challenge. Organizations managing diverse therapy portfolios must optimize operational efficiency to maintain sustainable economics.
PragLife addresses this challenge by:
- Reducing training requirements across different therapy types through intuitive interfaces
- Minimizing system maintenance overhead with unified platform architecture
- Enabling rapid deployment of new therapy protocols through configuration, not customization
- Streamlining regulatory documentation processes across all therapy types
Future-Proofing Through PragLife’s Flexibility
The FDA’s new Platform Technology Designation could speed up approvals for rare disease therapies, meaning organizations must prepare for even greater diversity in therapy types and regulatory requirements.
PragLife’s configurable architecture ensures organizations can evolve with the changing landscape rather than requiring complete system overhauls for each new therapy type.
Key PragLife Advantages for Managing Diversity:
- Configuration Flexibility: PragLife adapts to new therapy types without custom development
- Regulatory Adaptability: Built-in support for evolving FDA and GMP requirements
- Seamless Integration: PragLife connects with existing manufacturing and quality systems
- Proven Scalability: Handles increasing therapy diversity without performance degradation
- Rapid Training: Staff quickly adapt to new therapy protocols within PragLife’s intuitive interface
Implementation: PragLife’s Phased Approach
PragLife’s implementation methodology addresses therapeutic diversity from day one:
Phase 1: Core platform deployment with initial therapy configurations
Phase 2: Advanced workflow orchestration for multiple therapy types
Phase 3: Full ecosystem integration and optimization across all therapy protocols
This phased approach ensures organizations can begin managing their most critical therapy types immediately while scaling to handle increased diversity over time.
Conclusion: Transforming Complexity into Competitive Advantage
The diversity of cell and gene therapies isn’t a problem to be solved, it’s a reality to be managed intelligently. Organizations that embrace this complexity through PragLife’s configurable platform position themselves to capitalize on the expanding therapeutic landscape while maintaining the precision required for patient safety.
The question isn’t whether therapy diversity will continue increasing – with 51 percent of newly initiated gene therapy trials for non-oncology indications, expansion is inevitable. The question is whether organizations will manage this diversity through reactive complexity or proactive intelligence.
PragLife doesn’t eliminate the challenges of managing diverse cell and gene therapies. Instead, it transforms these challenges into manageable, scalable processes that adapt as the therapeutic landscape evolves, all while maintaining FDA compliance and GMP standards across every therapy type.
Ready to transform your cell and gene therapy operations? Experience how PragLife’s configurable platform can manage your most complex therapy diversity challenges. Schedule a demo to see PragLife in action and discover how leading organizations are turning therapeutic complexity into competitive advantage.



