Spreadsheets Vs. Sample Inventory Management Systems: A Comparative Guide for Managing Biological and Cell Therapy Sample Data
In the highly regulated world of life sciences and cell therapy, proper inventory management of biological samples isn’t just good practice it’s critical for patient safety, regulatory compliance, and operational success. Organizations working with biological materials face unique challenges that demand robust solutions beyond what conventional tracking methods can offer.
This guide provides a thorough comparison between traditional spreadsheet-based inventory management and specialized sample storage inventory systems designed specifically for GMP-regulated environments. Understanding these differences is crucial for organizations seeking to enhance their sample management processes while maintaining FDA compliance.
The Current Landscape of Sample Management in Life Sciences
Many cell therapy organizations, biobanks, and research institutions begin their journey using spreadsheets to track samples and inventory. While this approach might suffice initially, the increasing complexity of operations, stringent regulatory requirements, and the critical nature of biological materials demand more sophisticated solutions.
According to recent industry surveys, approximately 60% of small to mid-sized biopharmaceutical organizations still rely primarily on spreadsheets or basic database systems for sample tracking. However, as these organizations scale, the limitations become increasingly apparent and potentially hazardous.
Spreadsheets as Inventory Management Tools
Advantages of Spreadsheet-Based Systems
- Low barrier to entry: No additional software investment required initially
- Familiarity: Most staff already understand basic spreadsheet functions
- Flexibility: Can be quickly modified to accommodate new requirements
No implementation period: Can be created and deployed immediately
Limitations and Risks of Spreadsheet
- Manual data entry: Prone to human error with reported error rates of 1-5%
- Version control challenges: Multiple versions lead to data inconsistencies
- Limited audit trails: Difficulty tracking who made changes and when
- Inadequate security: Limited access controls and permission settings
- Scalability issues: Performance degrades as data volume increases
- Compliance challenges: Difficulty maintaining 21 CFR Part 11 compliance
- Search limitations: Inefficient retrieval of specific sample information
- No real-time updates: Potential for outdated information across teams
- Limited freezer visualization: No intuitive representation of storage locations
- Reporting constraints: Time-consuming manual report generation
These limitations create significant challenges for GMP-regulated environments where sample integrity, chain of custody, and regulatory compliance are paramount.
Using Dedicated Sample Inventory Management Systems
Purpose-built inventory management systems like PragLife’s Storage Inventory module are designed specifically to address the unique requirements of biological sample management in regulated environments.
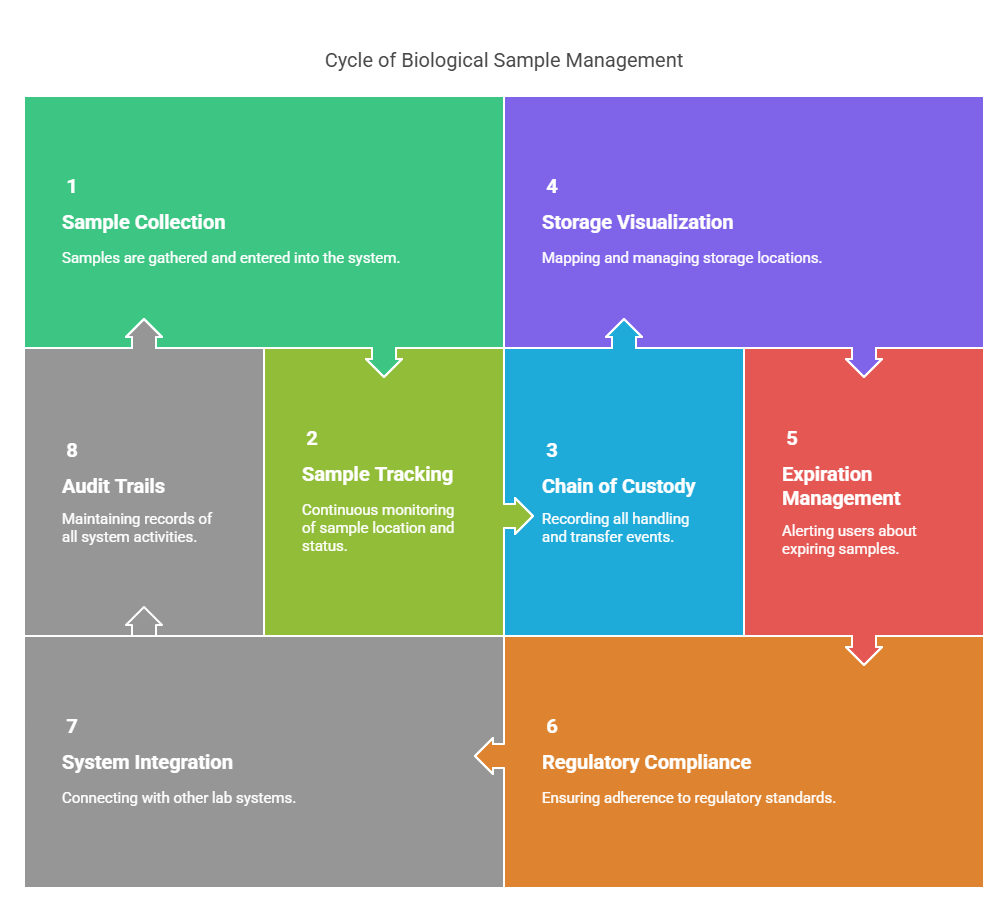
Key Features and Capabilities
- Comprehensive sample tracking: End-to-end visibility from collection to storage to usage
- Chain of custody documentation: Automatically records every transfer and handling instance
- Freezer mapping visualization: Intuitive interface that mirrors physical storage layouts
- Barcode/RFID integration: Eliminates manual data entry errors
- Regulatory compliance tools: Built-in features to support FDA audits and inspections
- Automated expiration management: Proactive alerts for expiring samples
- Role-based access controls: Granular permissions that enhance security
- Electronic signatures: 21 CFR Part 11 compliant authentication
- Complete audit trails: Immutable record of all system activities
- Integration capabilities: Connects with other laboratory systems (LIMS, ELN, etc.)
- Configurable workflows: Adaptable to specific organizational processes
- Robust search functionality: Quick retrieval of samples based on multiple parameters
Benefits for GMP Organizations
According to organizations that have transitioned from spreadsheets to dedicated systems:
- 85% reduction in sample location errors
- 75% decrease in time spent on inventory audits
- 90% improvement in regulatory compliance documentation
- 60% faster sample retrieval times
- Significant reduction in expired or lost samples
Regulatory Considerations in Sample Management
FDA Requirements and GMP Compliance
The FDA requires strict adherence to Good Manufacturing Practices (GMP) for organizations handling biological materials, particularly those intended for patient use. Key regulatory requirements include:
- Complete traceability of every sample throughout its lifecycle
- Validated systems with appropriate controls
- Comprehensive documentation of all inventory movements
- Regular reconciliation of physical inventory against records
- Proper handling of deviations and out-of-specification events
- Data integrity measures to prevent unauthorized changes
How Different Systems Support Compliance
Spreadsheet Limitations:
- Limited validation capabilities
- Difficult to maintain compliant audit trails
- Challenging to implement required security controls
- Manual reconciliation processes prone to error
- Time-consuming documentation for regulatory inspections
Dedicated System Advantages:
- Built-in compliance features aligned with 21 CFR Part 11
- Automated documentation generation for inspections
- Validated processes with proper change control
- Secure, tamper-evident audit trails
- Streamlined deviation management
Making the Transition: From Spreadsheets to Specialized Systems
Organizations considering the transition from spreadsheets to dedicated sample storage inventory systems should evaluate the following factors:
Key Evaluation Criteria
- Current and anticipated sample volume
- Regulatory requirements specific to your operations
- Integration needs with existing laboratory systems
- Budget constraints and ROI considerations
- Implementation timeline and resource availability
- Vendor experience with GMP environments
- Support for specific sample types and storage conditions
- Validation requirements and support
Implementation Best Practices
- Begin with a thorough analysis of current processes and pain points
- Define clear requirements before system selection
- Plan for comprehensive data migration from existing spreadsheets
- Allocate sufficient resources for validation activities
- Implement in phases to minimize operational disruption
- Provide thorough training to all system users
- Establish clear SOPs for the new system
Conclusion
While spreadsheets may seem sufficient for early-stage or small-scale operations, the risks they pose in terms of data integrity, regulatory compliance, and operational efficiency grow exponentially as organizations scale. The potential consequences – regulatory findings, compromised sample integrity, or even patient safety issues – far outweigh the initial investment in specialized sample management systems.
Dedicated inventory management solutions designed specifically for biological samples provide the robust features, compliance support, and operational efficiency that GMP-regulated organizations require. By implementing systems with purpose-built functionality for life sciences applications, organizations can enhance their sample management processes, ensure regulatory compliance, and ultimately focus on their core mission of advancing patient care through innovative therapies.
As the life sciences industry continues to evolve with increasingly complex therapies and stringent regulations, the tools used to manage critical samples must evolve as well. Making an informed decision about inventory management approaches is an essential step in building a foundation for sustainable growth and compliance in today’s challenging regulatory environment.



