Why Cell Therapy Supply Chains Need Cellular Orchestration Platforms
The cell and gene therapy (CGT) landscape has evolved dramatically over the past decade. With over 3,000 clinical trials worldwide and commercial therapies reaching patients at unprecedented speed, the industry faces a critical challenge orchestrating increasingly complex supply chains that span continents and involve multiple stakeholders.
Unlike traditional pharmaceuticals manufactured in standardized batches, cell therapies follow a personalized model where each treatment is unique to one patient. This fundamental difference creates cellular orchestration challenges that traditional supply chain management approaches simply cannot address.
The Complexity Challenge in Cell Therapy Supply Chains
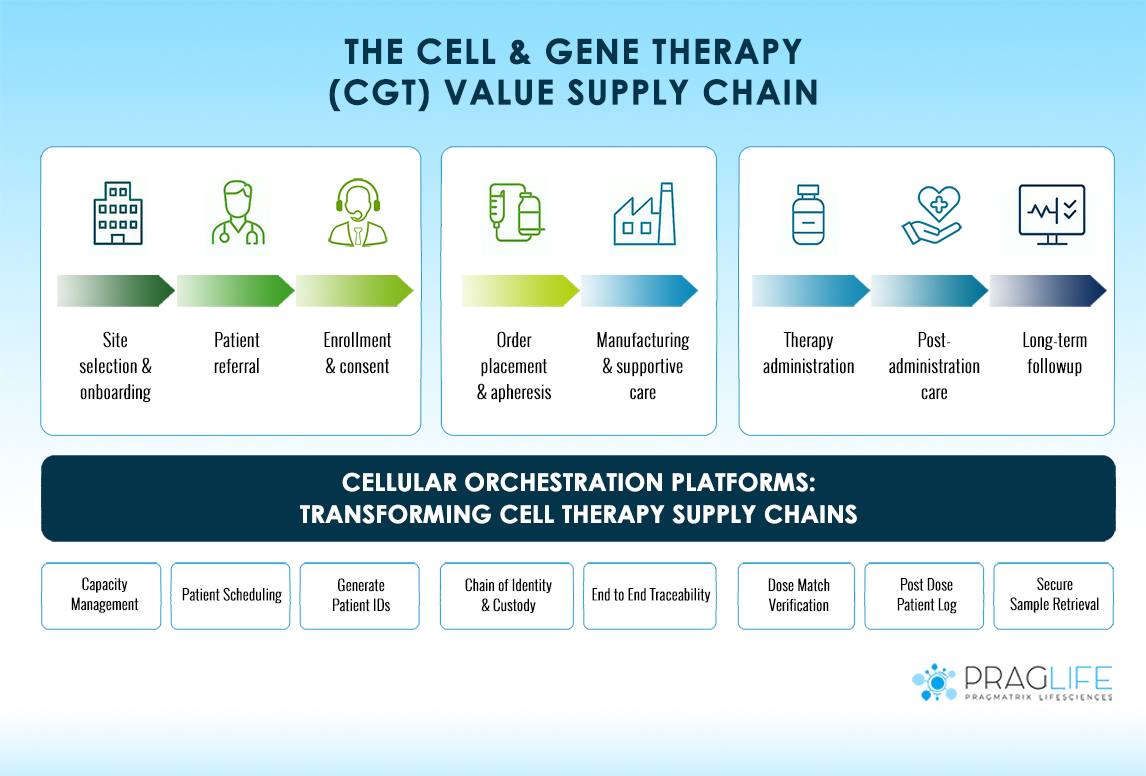
Cell therapy supply chains operate fundamentally differently from conventional pharmaceutical logistics. Consider the journey of a typical CAR-T therapy:
- Patient Assessment: Initial evaluation and enrollment at a treatment center
- Cell Collection: Apheresis procedure to harvest patient cells
- Shipping: Temperature-controlled transport to manufacturing facility
- Manufacturing: 2-3 week processing under strict GMP conditions
- Quality Testing: Comprehensive release testing and documentation
- Return Shipping: Transport back to treatment facility
- Patient Preparation: Conditioning regimen and infusion scheduling
- Administration: Final therapy delivery to patient
Each step involves different stakeholders, regulatory requirements, and critical timing considerations. A delay at any point can impact patient outcomes or result in product loss.
Recent industry data reveals that specimen loss rates hover around 0.002%, which translates to thousands of mishandled samples monthly across healthcare systems. For personalized therapies where replacement is impossible, these losses represent not just financial impact but potential patient harm.
Key Components of Effective Cellular Orchestration
Successful cell therapy orchestration requires several interconnected capabilities that work together as an integrated system:
1. Real-Time Visibility and Tracking
Modern orchestration platforms provide end-to-end visibility across the entire supply chain. This includes real-time location tracking, temperature monitoring, and status updates at every critical milestone. Implementing proper Chain of Identity (COI) and Chain of Custody (COC) tracking ensures complete traceability from patient to patient.
For example, platforms like PragLife’s Universal Scheduler coordinate complex procedures involving patients, hospitals, clinics, labs, and manufacturing plants across different time zones. When a patient’s apheresis is scheduled in New York, the system automatically reserves manufacturing capacity in a European facility, coordinates shipping logistics, and blocks infusion slots at the treating hospital.
2. Automated Scheduling and Coordination
Orchestration platforms coordinate complex scheduling across multiple facilities and time zones. This includes:
- Patient appointment scheduling
- Manufacturing slot allocation
- Shipping coordination with carriers
- Hospital bed and infusion suite reservations
- Staff scheduling and resource planning
The key differentiator lies in understanding the intricate relationships between different process steps, rather than treating them as isolated scheduling events.
3. Regulatory Compliance Management
GMP compliance and FDA regulations require comprehensive documentation throughout the manufacturing process. Orchestration platforms automate compliance activities including:
- Electronic batch record generation
- Deviation tracking and resolution
- Change control management
- Audit trail maintenance
- Regulatory reporting preparation
Leading platforms integrate these compliance requirements directly into workflows, ensuring Standard Operating Procedures (SOPs) are embedded in every process step rather than existing as separate documentation.
4. Quality Management Integration
Quality systems integration ensures that manufacturing data, environmental monitoring, and testing results flow seamlessly through the orchestration platform. This enables real-time quality decision-making and accelerates product release processes.
Technology Enablers for Cellular Orchestration
Several key technologies enable effective cellular orchestration platforms:
1. Barcode and RFID Integration
Implementing barcode and RFID technology eliminates manual data entry errors and provides automated tracking capabilities. These technologies enable instant product identification and status updates throughout the supply chain.
Successful implementations show that platforms with native barcode and RFID support significantly simplify deployment compared to systems requiring extensive customization. For instance, PragLife’s integrated Label Configurator and Carrier Connect modules demonstrate how embedded functionality streamlines both internal tracking and external logistics coordination.
2. Cloud-Based Architecture
Cloud platforms provide the scalability and accessibility needed for global orchestration. They enable real-time data sharing between facilities while maintaining security and compliance requirements.
3. API Integration Capabilities
Modern orchestration platforms integrate with existing hospital information systems, LIMS, ERP systems, and carrier tracking platforms. This eliminates data silos and ensures seamless information flow.
4. Mobile Applications
Mobile interfaces enable stakeholders to access critical information and update status from any location. This is particularly important for logistics personnel and clinical teams who need real-time access to product information.
Implementation Considerations Of Cellular Orchestration Platforms
Organizations evaluating cellular orchestration platforms should consider several key factors:
1. Build vs. Buy Decision
The choice between off-the-shelf solutions and custom development depends on organizational requirements, budget, and timeline constraints. While custom solutions offer maximum flexibility, pre-built platforms can accelerate implementation and reduce validation effort.
Many organizations find success with modular platforms that offer pre-built components while allowing configuration to specific workflows. This approach provides faster implementation than custom development while maintaining the flexibility needed for unique business processes.
2. Validation and Compliance Requirements
GMP software validation requires comprehensive documentation and testing. Organizations should plan for validation activities early in the implementation process and ensure their chosen platform provider has experience with FDA requirements.
Proper implementation guidance emphasizes the importance of partnering with vendors who understand both the technical requirements and regulatory landscape of cell therapy manufacturing.
3. Change Management and Training
Moving from manual processes to automated orchestration requires significant change management. Success depends on comprehensive training programs and user adoption strategies that address the cultural shift from paper-based to digital workflows.
4. Scalability Planning
Cell therapy volumes are growing rapidly as more therapies reach commercial scale. Orchestration platforms must be able to scale with business growth while maintaining performance and compliance standards.
Measuring Success: Key Performance Indicators
Organizations implementing cellular orchestration platforms typically track several key metrics:
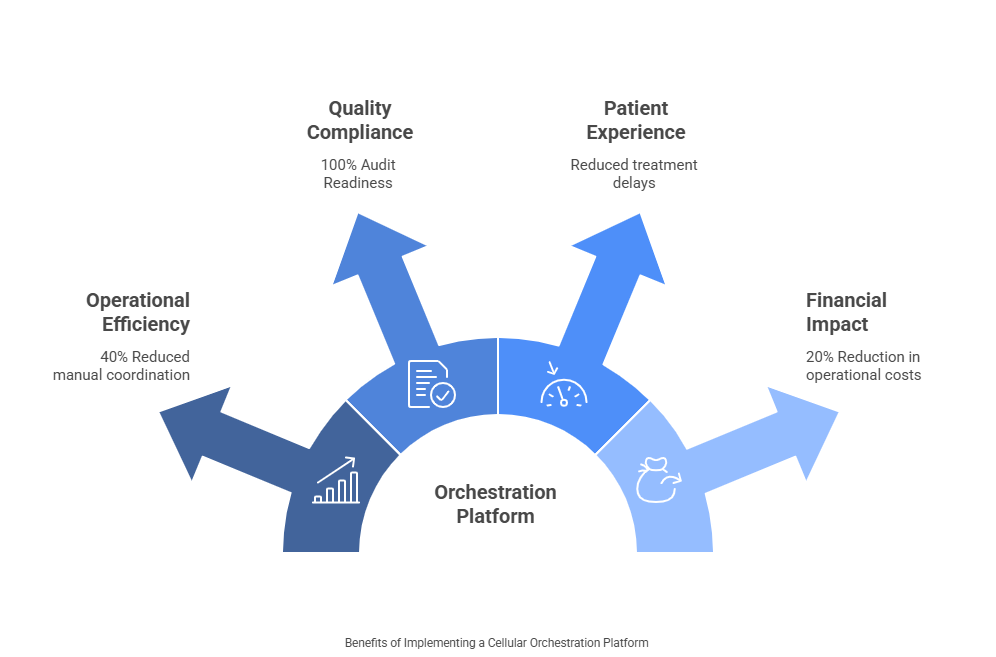
Operational Efficiency
- 40% reduction in manual coordination activities
- 60% faster batch record completion
- 25% improvement in resource utilization
Quality and Compliance
- 90% reduction in documentation errors
- 100% audit readiness
- Zero regulatory compliance issues
Patient Experience
- 30% reduction in treatment delays
- 95% patient satisfaction with scheduling coordination
- Improved communication throughout treatment journey
Financial Impact
- 20% reduction in operational costs
- 15% improvement in manufacturing yield
- Faster time-to-market for new therapies
These metrics demonstrate the tangible benefits that well-implemented orchestration platforms deliver across multiple organizational areas.
Future Trends in Cellular Orchestration
The cellular orchestration landscape continues to evolve with several emerging trends:
Artificial Intelligence Integration
AI and machine learning capabilities are being integrated to optimize scheduling, predict potential delays, and automate routine decision-making processes.
Blockchain Technology
Blockchain provides immutable audit trails and enhanced security for high-value cell therapies, particularly important for commercial products.
IoT and Smart Packaging
Internet of Things (IoT) sensors and smart packaging provide continuous monitoring of environmental conditions and product integrity throughout the supply chain.
Standardization Initiatives
Industry organizations are working to develop standards for data exchange and process orchestration to simplify multi-site coordination.
Conclusion
Cellular orchestration platforms represent a critical enabler for the growing cell and gene therapy industry. As therapy volumes increase and supply chains become more complex, organizations that master these orchestration capabilities will be best positioned for success.
The key to successful implementation lies in selecting platforms that address the unique requirements of cell therapy supply chains while providing the flexibility to adapt to evolving business needs. Organizations should evaluate their specific requirements, compliance obligations, and growth plans when selecting orchestration solutions.
Success factors include choosing platforms with native functionality for key requirements, ensuring proper validation support, and planning comprehensive change management programs. Those who invest in sophisticated orchestration capabilities today will define the standards for tomorrow’s cell therapy delivery.
Ready to explore how cellular orchestration can transform your operations? Schedule a 45-minute discovery session to discuss your specific challenges and requirements with our team of experts.



